Sep 8, 2023Instant Answer EXPERT VERIFIED Step 1/3 Step 1: Determine the molar mass of calcium. The molar mass of calcium (Ca) is 40.08 g/mol. Step 2/3 Step 2: Calculate the number of moles of calcium in 143 g. Number of moles = mass / molar mass Number of moles = 143 g / 40.08 g/mol Number of moles = 3.567 mol Answer
Answered: How many moles of calcium atoms do you… | bartleby
Avogadro’s number is thus the link between the macro world of grams and kilograms, that which we can directly measure, and the micro world of atoms and molecules, about which we can conceive. So moles of Ca = 183.08 ⋅ g 40.078 ⋅ g ⋅ mol−1 = approx. 4.5 ⋅ mol. Number of calcium atoms = 4.5 ⋅ mol ×6.02247 ×1023 ⋅ mol−1 = ??how many calcium atoms

Source Image: mdpi.com
Download Image
Suppose you have 10 gram of carbon. Now, it should be converted in terms of atoms. We know that the molar mass of 1 mole of carbon is equal to 12. It means that 12 grams per mole. Also, the Avogadro constant is 6.02 x 10 23. So, the conversion of carbon from grams to atoms is given by = 10(1/12)( 6.02 x 10 23) = 5.02 x 10 23

Source Image: ahajournals.org
Download Image
The Atomic-Level Structure of Cementitious Calcium Aluminate Silicate Hydrate | Journal of the American Chemical Society
1 Answer Nathan L. Jun 7, 2017 2.30 ×1024 atoms Ca Explanation: We’re asked to calculate the number of atoms of Ca in 153 g Ca. What we must first do is convert the given mass of calcium to moles of calcium, using its molar mass (referring to a periodic table, this is 40.08 g mol ): 153 g Ca( 1mol Ca 40.08g Ca) = 3.82 mol Ca
Source Image: quora.com
Download Image
How Many Atoms Are In 143 G Of Calcium
1 Answer Nathan L. Jun 7, 2017 2.30 ×1024 atoms Ca Explanation: We’re asked to calculate the number of atoms of Ca in 153 g Ca. What we must first do is convert the given mass of calcium to moles of calcium, using its molar mass (referring to a periodic table, this is 40.08 g mol ): 153 g Ca( 1mol Ca 40.08g Ca) = 3.82 mol Ca
About this tutor ›. Avogadro’s number = 6.02×10 23 particles per mol (particles can be atoms, molecules, formula units, etc) atomic mass Ca = 40.08 g / mol. moles Ca = 3456 g x 1 mol / 40.08 g = 86.228 moles. atoms of Ca = 82.228 moles x 6.02×10 23 atoms / mol = 519.1×10 23 atoms = 5.191×10 25 atoms of Ca. ANSWER (a)
How many atoms are present in 0.6gram atom of calcium? – Quora
Therefore, 143 grams of calcium contain: N = 143 g × 6.022 × 1 0 23 m o l − 1 40.08 g m o l = 2.15 × 1 0 24 \beginaligned N&=\frac\pu143 g \times 6.022 \times 10^23 mol^-1\pu40.08 \fracgmol\\ &=\pu2.15 \times 10^24 \endaligned N = 40.08 mol g 143 g × 6.022 × 1 0 23 mo l − 1 = 2.15 × 1 0 24 2.15 × \times × 10 24
Chemistry IB P.1.15 How many atoms are present in 2 grams of calcium? (GAW of Ca is 40g) Solution 40 orams of ca has 6022 x 1023 atoms

Source Image: toppr.com
Download Image
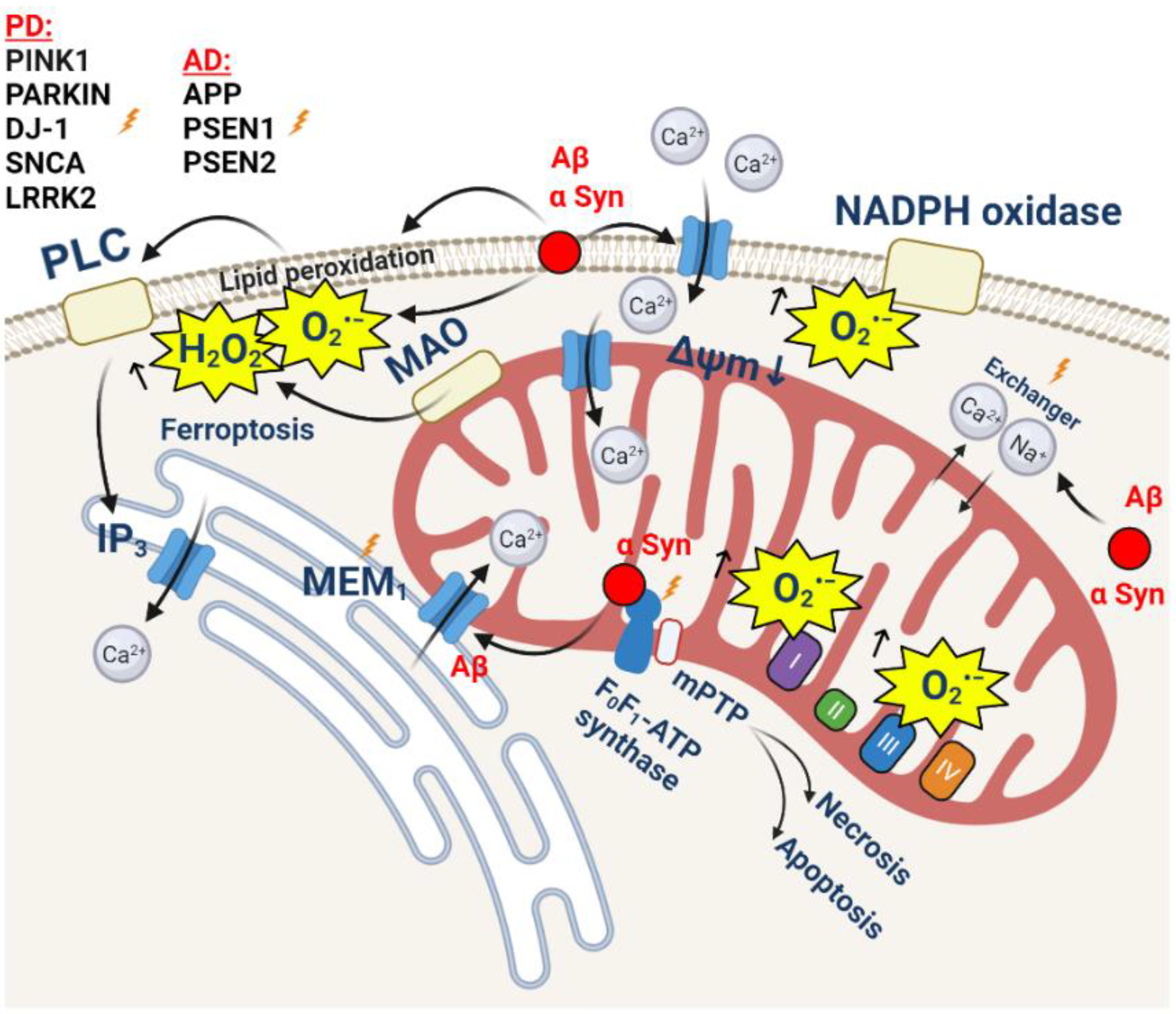
Cells | Free Full-Text | Interaction of Mitochondrial Calcium and ROS in Neurodegeneration
Therefore, 143 grams of calcium contain: N = 143 g × 6.022 × 1 0 23 m o l − 1 40.08 g m o l = 2.15 × 1 0 24 \beginaligned N&=\frac\pu143 g \times 6.022 \times 10^23 mol^-1\pu40.08 \fracgmol\\ &=\pu2.15 \times 10^24 \endaligned N = 40.08 mol g 143 g × 6.022 × 1 0 23 mo l − 1 = 2.15 × 1 0 24 2.15 × \times × 10 24
Source Image: mdpi.com
Download Image
Answered: How many moles of calcium atoms do you… | bartleby
Sep 8, 2023Instant Answer EXPERT VERIFIED Step 1/3 Step 1: Determine the molar mass of calcium. The molar mass of calcium (Ca) is 40.08 g/mol. Step 2/3 Step 2: Calculate the number of moles of calcium in 143 g. Number of moles = mass / molar mass Number of moles = 143 g / 40.08 g/mol Number of moles = 3.567 mol Answer

Source Image: bartleby.com
Download Image
The Atomic-Level Structure of Cementitious Calcium Aluminate Silicate Hydrate | Journal of the American Chemical Society
Suppose you have 10 gram of carbon. Now, it should be converted in terms of atoms. We know that the molar mass of 1 mole of carbon is equal to 12. It means that 12 grams per mole. Also, the Avogadro constant is 6.02 x 10 23. So, the conversion of carbon from grams to atoms is given by = 10(1/12)( 6.02 x 10 23) = 5.02 x 10 23

Source Image: pubs.acs.org
Download Image
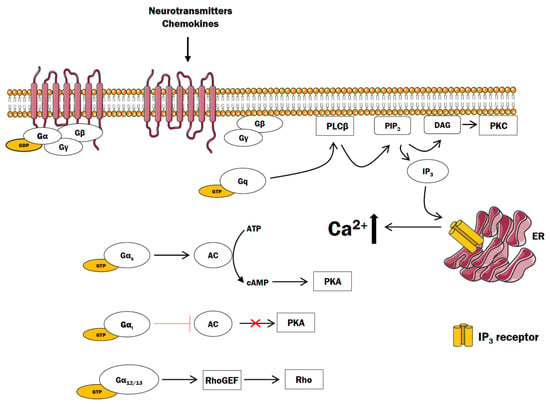
Cells | Free Full-Text | The Role of G Protein-Coupled Receptors (GPCRs) and Calcium Signaling in Schizophrenia. Focus on GPCRs Activated by Neurotransmitters and Chemokines
Science How many atoms are in 143 g of calcium? Question: How many atoms are in 143 g of calcium? Mole: The mole is a unit that expresses the number of atoms, molecules, or
Source Image: mdpi.com
Download Image
Nanoparticle platforms for dermal antiaging technologies: Insights in cellular and molecular mechanisms – Bhatia – 2022 – WIREs Nanomedicine and Nanobiotechnology – Wiley Online Library
1 Answer Nathan L. Jun 7, 2017 2.30 ×1024 atoms Ca Explanation: We’re asked to calculate the number of atoms of Ca in 153 g Ca. What we must first do is convert the given mass of calcium to moles of calcium, using its molar mass (referring to a periodic table, this is 40.08 g mol ): 153 g Ca( 1mol Ca 40.08g Ca) = 3.82 mol Ca

Source Image: wires.onlinelibrary.wiley.com
Download Image
29Si NMR in Cement: A Theoretical Study on Calcium Silicate Hydrates | The Journal of Physical Chemistry C
About this tutor ›. Avogadro’s number = 6.02×10 23 particles per mol (particles can be atoms, molecules, formula units, etc) atomic mass Ca = 40.08 g / mol. moles Ca = 3456 g x 1 mol / 40.08 g = 86.228 moles. atoms of Ca = 82.228 moles x 6.02×10 23 atoms / mol = 519.1×10 23 atoms = 5.191×10 25 atoms of Ca. ANSWER (a)

Source Image: pubs.acs.org
Download Image
Cells | Free Full-Text | Interaction of Mitochondrial Calcium and ROS in Neurodegeneration
29Si NMR in Cement: A Theoretical Study on Calcium Silicate Hydrates | The Journal of Physical Chemistry C
Avogadro’s number is thus the link between the macro world of grams and kilograms, that which we can directly measure, and the micro world of atoms and molecules, about which we can conceive. So moles of Ca = 183.08 ⋅ g 40.078 ⋅ g ⋅ mol−1 = approx. 4.5 ⋅ mol. Number of calcium atoms = 4.5 ⋅ mol ×6.02247 ×1023 ⋅ mol−1 = ??how many calcium atoms
The Atomic-Level Structure of Cementitious Calcium Aluminate Silicate Hydrate | Journal of the American Chemical Society Nanoparticle platforms for dermal antiaging technologies: Insights in cellular and molecular mechanisms – Bhatia – 2022 – WIREs Nanomedicine and Nanobiotechnology – Wiley Online Library
Science How many atoms are in 143 g of calcium? Question: How many atoms are in 143 g of calcium? Mole: The mole is a unit that expresses the number of atoms, molecules, or