To balance a redox equation using the half-reaction method, the equation is first divided into two half-reactions, one representing oxidation and one representing reduction. The equations for the half-reactions are then balanced for mass and charge and, if necessary, adjusted so that the number of electrons transferred in each equation is the same.
Electrochemistry Chapter 19 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. – ppt download
Nov 11, 20231 Expert Answer Best Newest Oldest J.R. S. answered • 11/11/23 Tutor 5.0 (145) Ph.D. University Professor with 10+ years Tutoring Experience About this tutor › 2 Fe 3+ + 1 NO 2- + 1 H 2 O ==> 2 Fe 2+ + 2 H + + 1 NO 3- Oxidation half reaction: NO 2- ===> NO 3- NO 2- + H 2 O ===> NO 3- .. balanced for N and O

Source Image: youtube.com
Download Image
Contributors; In studying redox chemistry, it is important to begin by learning to balance electrochemical reactions. Simple redox reactions (for example, H 2 + I 2 → 2 HI) can be balanced by inspection, but for more complex reactions it is helpful to have a foolproof, systematic method. The electron-ion method allows one to balance redox reactions regardless of their complexity.

Source Image: youtube.com
Download Image
39: Balancing redox reactions – YouTube Lesson 3: Balancing redox reactions. Balancing redox equations. Worked example: Balancing a simple redox equation. Worked example: Balancing a redox equation in acidic solution. Worked example: Balancing a redox equation in basic solution. Oxidation-reduction (redox) reactions. Balancing redox reactions.

Source Image: scribd.com
Download Image
Balance The Redox Reaction By Inserting The Appropriate Coefficients.
Lesson 3: Balancing redox reactions. Balancing redox equations. Worked example: Balancing a simple redox equation. Worked example: Balancing a redox equation in acidic solution. Worked example: Balancing a redox equation in basic solution. Oxidation-reduction (redox) reactions. Balancing redox reactions. Nov 14, 2023Redox reactions are most easily balanced by splitting them into two “half reactions,” balancing the half reactions, and then adding them together to get the complete balanced equation. In this case it’s most obvious that Cd is gaining electrons because it goes from being +2 to neutral. In order to do that it must have gained two electrons.
Redox Reactions (Theory) Edited | PDF | Redox | Electrochemistry
Aug 29, 2023Solution. Step 1: Separate the half-reactions. By searching for the reduction potential, one can find two separate reactions: Cu + (aq) + e − → Cu(s) and. Fe3 + (aq) + 3e − → Fe(s) The copper reaction has a higher potential and thus is being reduced. Iron is being oxidized so the half-reaction should be flipped. Balancing Redox Reactions in Basic Medium. Chemistry Class with Elvis Adobah. Easy understanding. – YouTube

Source Image: m.youtube.com
Download Image
Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry – YouTube Aug 29, 2023Solution. Step 1: Separate the half-reactions. By searching for the reduction potential, one can find two separate reactions: Cu + (aq) + e − → Cu(s) and. Fe3 + (aq) + 3e − → Fe(s) The copper reaction has a higher potential and thus is being reduced. Iron is being oxidized so the half-reaction should be flipped.
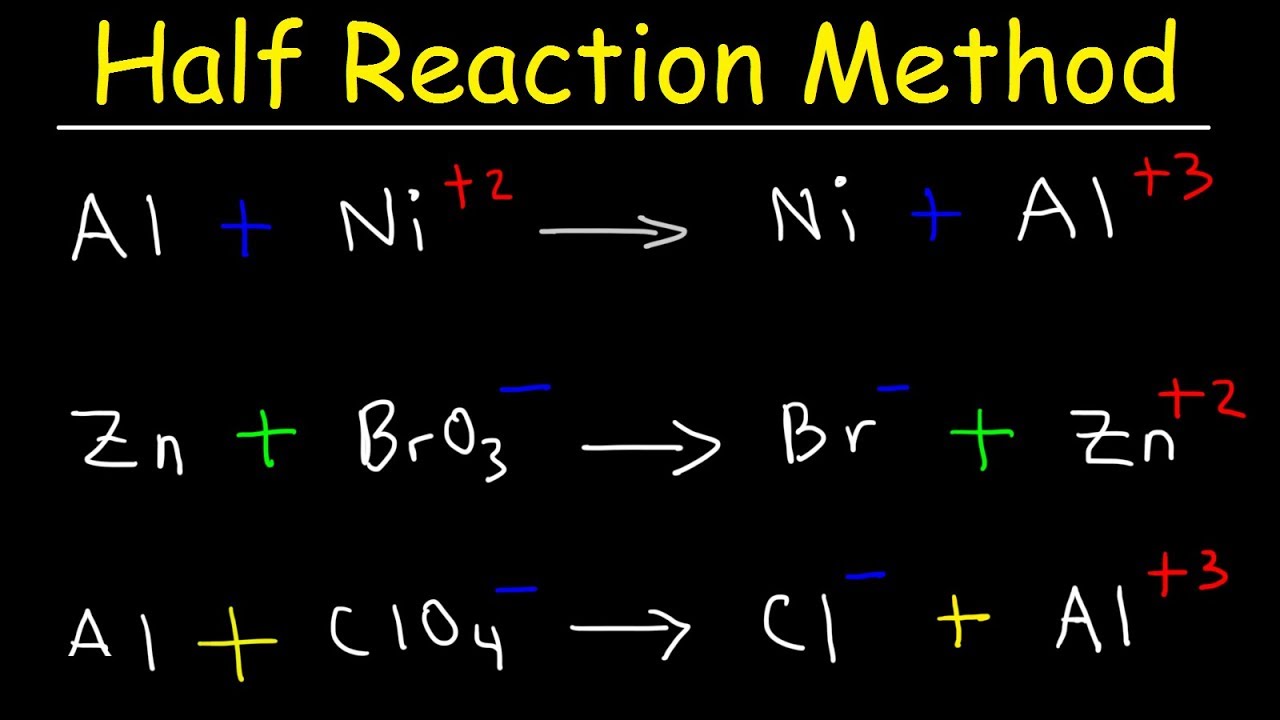
Source Image: m.youtube.com
Download Image
Electrochemistry Chapter 19 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. – ppt download To balance a redox equation using the half-reaction method, the equation is first divided into two half-reactions, one representing oxidation and one representing reduction. The equations for the half-reactions are then balanced for mass and charge and, if necessary, adjusted so that the number of electrons transferred in each equation is the same.

Source Image: slideplayer.com
Download Image
39: Balancing redox reactions – YouTube Contributors; In studying redox chemistry, it is important to begin by learning to balance electrochemical reactions. Simple redox reactions (for example, H 2 + I 2 → 2 HI) can be balanced by inspection, but for more complex reactions it is helpful to have a foolproof, systematic method. The electron-ion method allows one to balance redox reactions regardless of their complexity.

Source Image: m.youtube.com
Download Image
Redox Reactions (Theory) Edited | PDF | Redox | Electrochemistry About Transcript A redox equation can be balanced using the following stepwise procedure: (1) Divide the equation into two half-reactions. (2) Balance each half-reaction for mass and charge. (3) Equalize the number of electrons transferred in each half-reaction. (4) Add the half-reactions together.

Source Image: scribd.com
Download Image
SOLUTION: balancing redox reactions by ion electron method – Studypool Lesson 3: Balancing redox reactions. Balancing redox equations. Worked example: Balancing a simple redox equation. Worked example: Balancing a redox equation in acidic solution. Worked example: Balancing a redox equation in basic solution. Oxidation-reduction (redox) reactions. Balancing redox reactions.

Source Image: studypool.com
Download Image
Balancing Redox Reactions Using Oxidation Numbers Grade 12 Chemistry Power Point WITH ANSWERS 21PGS | Teaching Resources Nov 14, 2023Redox reactions are most easily balanced by splitting them into two “half reactions,” balancing the half reactions, and then adding them together to get the complete balanced equation. In this case it’s most obvious that Cd is gaining electrons because it goes from being +2 to neutral. In order to do that it must have gained two electrons.

Source Image: tes.com
Download Image
Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry – YouTube
Balancing Redox Reactions Using Oxidation Numbers Grade 12 Chemistry Power Point WITH ANSWERS 21PGS | Teaching Resources Nov 11, 20231 Expert Answer Best Newest Oldest J.R. S. answered • 11/11/23 Tutor 5.0 (145) Ph.D. University Professor with 10+ years Tutoring Experience About this tutor › 2 Fe 3+ + 1 NO 2- + 1 H 2 O ==> 2 Fe 2+ + 2 H + + 1 NO 3- Oxidation half reaction: NO 2- ===> NO 3- NO 2- + H 2 O ===> NO 3- .. balanced for N and O
39: Balancing redox reactions – YouTube SOLUTION: balancing redox reactions by ion electron method – Studypool About Transcript A redox equation can be balanced using the following stepwise procedure: (1) Divide the equation into two half-reactions. (2) Balance each half-reaction for mass and charge. (3) Equalize the number of electrons transferred in each half-reaction. (4) Add the half-reactions together.